 |
||||||||||||||||
| Updated on August 19, 2013 | ||||||||||||||||
| 科学研究費補助金 基盤研究(B)プロジェクト (平成23~26年度) 「擬ブルッカイト型構造を有する低熱膨張・環境調和型セラミックス多孔体の応用」 |
||||||||||||||||
| Grant-in-Aid for Science Research No. 23350111 for Basic Research: Category B, JSPS/MEXT | ||||||||||||||||
 |
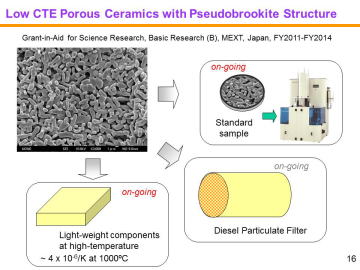
Uniformly Porous MgTi2O5 with Narrow Pore-Size Distribution | |||||||||||||||
 |
Diesel particulate filters (DPFs), typically made of porous ceramics, are widely used for trapping particulate matter (PM) in the diesel exhaust. Major ceramic companies, such as NGK, Ibiden and Corning, have mass-produced high-performance DPFs. At present, cordierite (Mg2Al4Si5O18) and silicon carbide (SiC) are used for DPF materials; the first generation, cordierite, is characterized by its low-thermal expansion, low-cost and light-weight, while the second generation, SiC, is featured by its high-strength, high refractoriness, high thermal conductivity and so on (suitable for high-speed vehicles).Corresponding to a growing diversity of biodiesel fuels and to severer environmental regulations, third-generation DPFs materials with low-thermal expansion, low-cost, high strength and high refractoriness will be demanded in the future. | |||||||||||||||
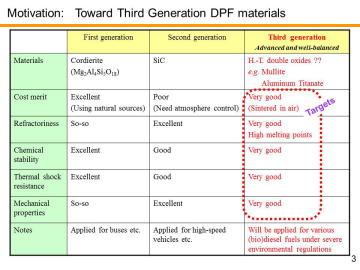 |
Several double oxides, such as mullite (3Al2O3·2SiO2), zircon (ZrSiO4) and aluminum titanate (Al2TiO5), are of particular interest as candidates of the third generation, due to their low thermal expansion coefficients among oxides. | |||||||||||||||
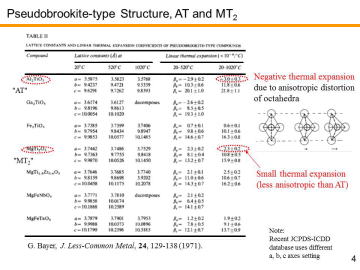 |
Among these candidates, Al2TiO5 (AT) has been eagerly studied due to its high thermal shock resistance
caused by the low-thermal expansion. Al2TiO5
has orthorhombic pseudobrookite-type crystal structure (generally expressed as
Me3O5). US Corning and Japanese Ohcera Co./Sumitomo
Chemical Co. are developing Al2TiO5-based DPFs. |
|||||||||||||||
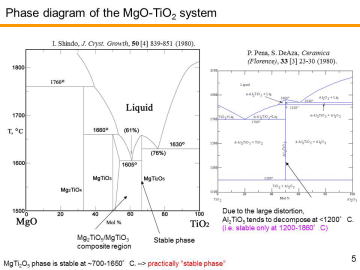 |
Although Al2TiO5 has good thermal shock resistance and relatively high melting point, undoped Al2TiO5 tends to decompose into Al2O3 and TiO2 at elevated temperatures. Due to the large distortion of MeO6-octahedra, Al2TiO5 phase gradually decomposes into Al2O3 and TiO2 under the high-temperature use below 1200 °C. | |||||||||||||||
 |
Furthermore, due to the strong anisotropy of thermal
expansion, resultant microcracks gradually degrades mechanical properties.
Hence, instead of pure Al2TiO5, Al2TiO5-based
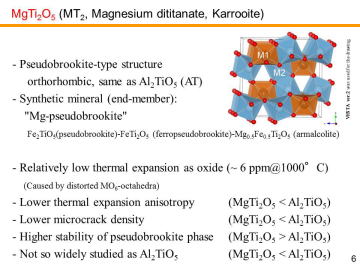
composites or solid solutions have been actually developed for DPF applications. MgTi2O5 (MT2) also has orthorhombic pseudobrookite-type crystal structure and also shows low thermal expansion. However, the thermal-expansion anisotropy of MgTi2O5 is not so prominent as Al2TiO5. MgTi2O5 is thermally more stable than Al2TiO5; it is reported that even after the heat-treatment at 700 °C for 162 h, crystal structure of the MgTi2O5 remained as pseudobrookite. Hence, MgTi2O5 has been thought as a “pseudobrookite-phase stabilizer” for Al2TiO5 phase to make Al2TiO5-MgTi2O5 solid solutions. Actually, Kyocera Co. has proposed MgTi2O5-Al2TiO5 DPFs.24) Despite its prospective characteristics, MgTi2O5 is not so widely studied as Al2TiO5. |
|||||||||||||||
 |
Thinking about its low-cost, safe, and refractory
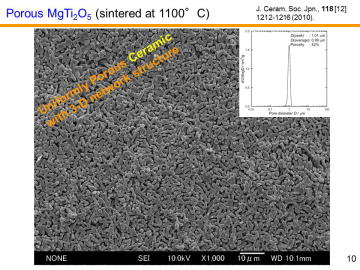
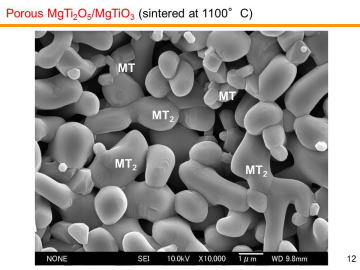
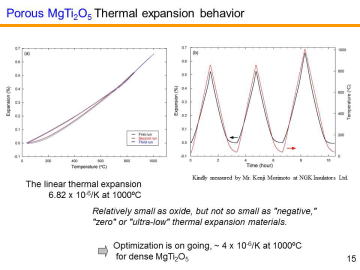
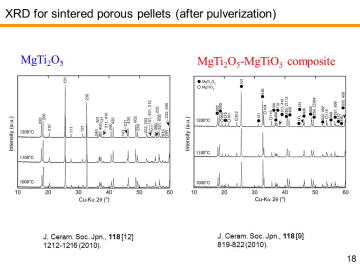
composition, MgTi2O5 can be another candidate for the third-generation DPF material. We have recently reported in situ processing and microstructure of uniformly porous MgTi2O5. Uniformly porous MgTi2O5 ceramics with very narrow pore size distribution have been synthesized by one-step pyrolytic reactive sintering (in situ processing), where decomposed CO2 gas from a carbonate source acts as an intrinsic pore forming agent. In order to control the pore-size distribution, to reduce the resource cost (i.e., using less TiO2 and more MgO), and to add some functions, MgTi2O5-MgTiO3 pseudobinary system seems to be attractive. |
|||||||||||||||
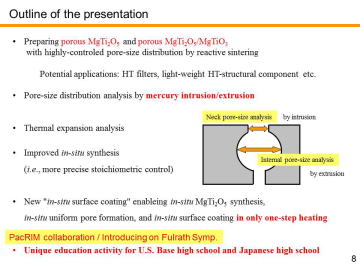 |
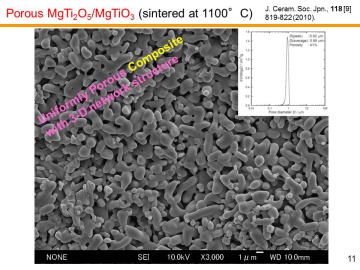
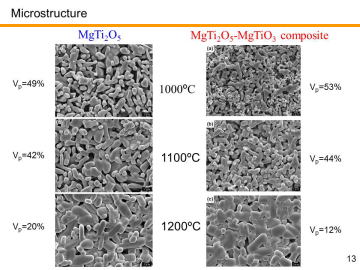
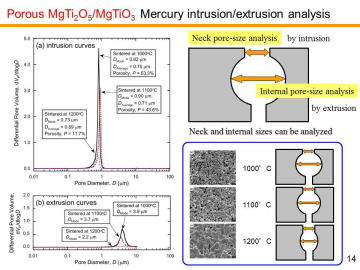
Porous MgTi2O5/MgTiO3 composites have been prepared by in situ processing at 1000-1200ºC. Porous MgTi2O5/MgTiO3 composites with very narrow pore-size distribution at the diameter of ~1 mm were obtained by the pyrolytic reactive sintering at 1000-1100ºC. Internal pore-structure was characterized by using mercury intrusion-extrusion porosimetry. | |||||||||||||||
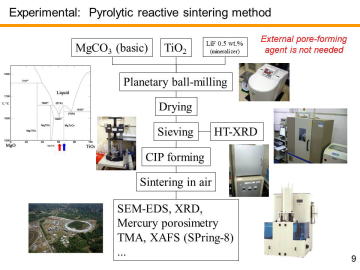 |
Similarly to previously reported UPC-3D (uniformly porous ceramic/composite
with three dimension network structure), magnesium carbonate is used as
a starting materials; decomposed CO2 gas, emitted during the one-step pyrolytic reacting sintering, forms very
uniform open porous structure. Commercially available MgCO3 (basic) powder (approx. 3MgCO3-Mg(OH)2-3H2O in catalog, 99.9% purity, Kojundo Chemical Laboratory Co. Ltd., Saitama, Japan), TiO2 anatase powder (99.9% purity, Kojundo Chemical Laboratory Co. Ltd.), and LiF powder (99.9%, Wako Pure Chemical Ind., Ltd, Osaka, Japan) were used as the starting materials. X-ray diffraction analysis revealed that the commercial MgCO3 (basic) powder was composed of hydromagnesite phase (Mg5(CO3)4(OH)2·4H2O, ICDD-JCPDS PDF 25-0513). LiF acts as a mineralizer. Note that low-cost natural resources may be replaceable for magnesium carbonate and TiO2. It is an advantage of MgO-TiO2 system. |
|||||||||||||||
 |
MgCO3 (basic) and TiO2 powders
(Mg:Ti = 1:2 in mole fraction) with LiF (0.5 mass% for total starting powders)
were wet-ball milled in ethanol for 2 h in a planetary ball-mill (acceleration:
4g). The mixed slurry was dried and then sieved through a 150-mesh screen.
To obtain bulk porous MgTi2O5, the mixed powder was cold isostatically pressed at 200 MPa after mold-pressing. The green compacts with no binder, ~15 mm in diameter and ~3 mm in thickness, were sintered in air at 1000-1200°C for 2 h to obtain the porous MgTi2O5. |
|||||||||||||||
 |
MgCO3 and TiO2 powders (Mg:Ti =
39:61 in mole fraction, corresponding to the eutectic composition of MgTi2O5-MgTiO3)
with LiF (0.5 mass% for total starting powders) were wet-ball milled in ethanol
for 2 h in a planetary ball-mill (acceleration: 4g). The mixed slurry
was dried and then sieved through a 150-mesh screen. To obtain bulk porous MgTi2O5/MgTiO3, the mixed powder was cold isostatically pressed at 200 MPa after mold-pressing. The green compacts with no binder, 15 mm in diameter and ~ 3 mm in thickness (cylinder), were sintered in air at 1000-1200°C for 2 h to obtain the porous MgTi2O5/MgTiO3. |
|||||||||||||||
 |
||||||||||||||||
 |
||||||||||||||||
 |
||||||||||||||||
 |
||||||||||||||||
 |
||||||||||||||||
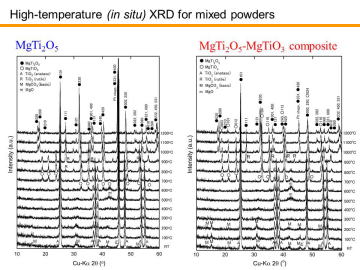 |
Left figure shows HT-XRD patterns for the MgCO3 (basic) and TiO2 mixed powder (Mg:Ti = 1:2 in mole fraction) doped with LiF (0.5 mass% for total starting powders). From room temperature to ~ 300°C, only hydromagnesite (i.e., basic MgCO3) and TiO2 anatase phases were confirmed. Around 400-500ºC, MgCO3 started to decompose into MgO fine particles (see a broad peak 2q~43º, corresponding to MgO(200)). At ~600ºC, intermediate MgTiO3 phase began to form, and it was clearly observed at 700-900ºC. At 800-900ºC, un-reacted TiO2 anatase partially converted to TiO2 rutile phase. At 1000ºC, formation of MgTi2O5 phase became prominent. At 1100-1200ºC, almost single-phase MgTi2O5 was obtained. This HT-XRD study suggested that porous MgTi2O5 can be obtained for sintering temperatures of 1000-1200ºC. Formation temperature of MgTi2O5 phase (~ 1000ºC) is in good agreement with a reported value in the literature. | |||||||||||||||
 |
||||||||||||||||
 |
||||||||||||||||
 |
The Ti K edge XAFS were recorded at room temperature at BL14B2 of SPring-8 in Japan. The Ti K edge XAFS data for this study were corrected with transmission mode using Si(111) double-crystal monochromator. Samples for XAFS measurement (pellets with ~0.5 mm thickness) were prepared by mixing of the above sample powders and a commercial BN powder (for dilution purpose), and then by uniaxially pressing. Metallic Ti foil was used for the energy calibration. Fourier transformation of the k3-weighted EXAFS oscillation from k space to r space was carried out to obtain the radial distribution function. Athena software was used for the data-processing. | |||||||||||||||
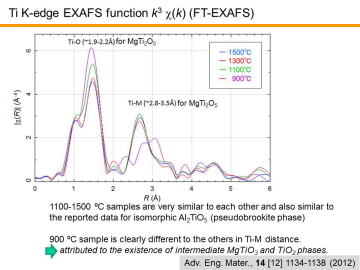 |
The figure shows the Fourier transform of Ti K-edge EXAFS function k3
c(k) (FT-EXAFS). In the figure, atomic
distances of Ti-O and Ti-M (metallic ion) of MgTi2O5
phase in the literature are also given. Note that the peak position of radial
distribution function calculated by FT-EXAFS is usually 0.2-0.5Å smaller than that
of the real atomic distance determined by XRD, due to the "phase"
difference of Fourier transform (here, "phase" in oscillation). In
our experiment, such phenomenon was observed. FT-EXAFS curves for 1100-1500 ºC are very similar to each other and also similar to the reported data for isomorphic Al2TiO5 (pseudobrookite phase). Meanwhile, FT-EXAFS curve for 900 ºC is clearly different to the others in Ti-M distance. The difference can be attributed to the existence of intermediate MgTiO3 and TiO2 phases. FT-EXAFS was sensitive for the detection of these intermediate phases. |
|||||||||||||||
 |
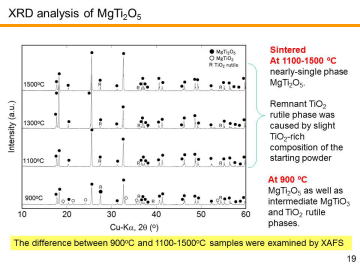
As discussed above, the chemical composition of the basic MgCO3 powder used in this study affected the final composition. In order to achieve more precise in-situ synthesis, XRD and TG-DTA analysis of the basic MgCO3 powder was carried out. | |||||||||||||||
 |
||||||||||||||||
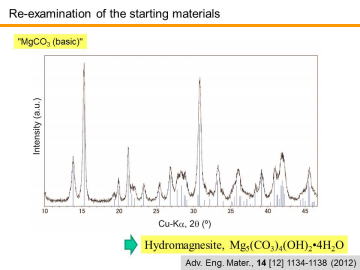 |
XRD analysis revealed that the commercial basic MgCO3 powder used in this study was actually composed of hydromagnesite phase, Mg5(CO3)4(OH)2•4H2O (i.e. 4MgCO3• Mg(OH)2•4H2O). | |||||||||||||||
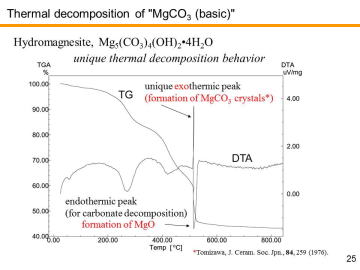 |
TG-DTA analysis revealed that nearly 60% weight loss was observed for the heating up to 1000 ºC. We have performed the TG-DTA analysis several times for this basic MgCO3 powder. Similar analysis was also conducted for the TiO2 anatase powder (for this powder, weight loss was only ~0.3%). By using these values, initial composition was calibrated and mixed powder was prepared. | |||||||||||||||
 |
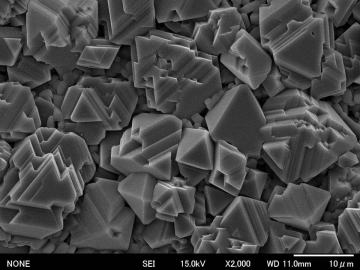
The figure shows a SEM photograph of porous MgTi2O5 sintered at 1200 ºC by using the calibrated starting powder. More faceted, rectangular-like crystals were formed compared with those of our previous research. | |||||||||||||||
 |
Thinking about the practical applications such as light weight structural materials or filter materials, porous materials with supporting surface layer (or coating) are favorable in some cases. In this study, we have tried to in-situ coat the surface of porous MgTi2O5 by porous MgAl2O4 spinel layer; MgAl2O4 spinel phase generally shows high chemical stability and good mechanical properties. The idea is very simple and practical. When the green pellets are set in sintering supports, dense alumina plates are placed in contact with the pellets. | |||||||||||||||
 |
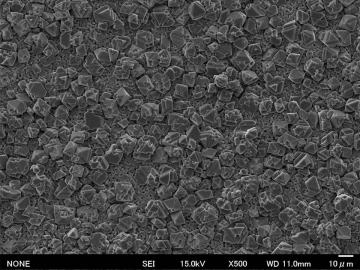
The figure shows SEM micrograph of the porous MgTi2O5 sintered at 1200 ºC by using the calibrated starting powder, covered by well-faceted porous MgAl2O4 spinel phase (low magnification and its enlargement). In our sintering process, LiF is used as a mineralizer, which accelerates the solid-state reactions, and then, most part of LiF escapes to the outside. When we cover the green pellets with dense alumina plate, LiF vapor stays at the MgTi2O5-alumina plate interface, and it may accelerate the reaction between MgO and Al2O3 at the pellet surface during the sintering. | |||||||||||||||
 |
Hence, in this case, in-situ MgTi2O5 synthesis, in-situ uniform pore formation (see the beneath of coating layer), and in-situ surface coating were achieved in only one-step heating. | |||||||||||||||
 |
||||||||||||||||
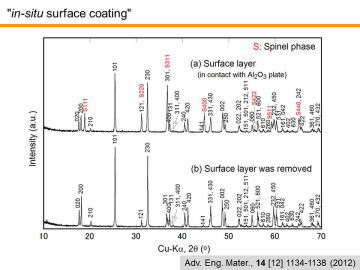 |
As for the main bulk phase, MgTi2O5, might be also doped with Al2O3, since MgTi2O5 can make the solid solution with isomorphic Al2TiO5. The figure represents the XRD patterns of the porous MgTi2O5 with in-situ surface coating: (a) surface layer, and (b) surface layer was removed. As can be seen in Fig. 8(a), spinel phase was found in the surface. When the surface was removed (just by scratching with sandpaper), single-phase MgTi2O5 (without remnant TiO2 rutile) was observed as described in the above section. | |||||||||||||||
| Y. Suzuki, and M. Morimoto, "Porous
MgTi2O5/MgTiO3 Composites with Narrow Pore-Size Distribution: In Situ
Processing and Pore Structure Analysis,"J. Ceram. Soc. Jpn., 118 [9] 819-822 (2010). Y. Suzuki and M. Morimoto, "Uniformly Porous MgTi2O5 with Narrow Pore-Size Distribution: In Situ Processing, Microstructure and Thermal Expansion Behavior," J. Ceram. Soc. Jpn., 118 [12] 1212-1216 (2010). Y. Suzuki and Y. Shinoda, "Magnesium Dititanate (MgTi2O5) with Pseudobrookite Structure: A Review," Sci. Tech. Adv. Mater., 12 [3] no.034301 (2011). Y. Suzuki, T. S. Suzuki, Y. Shinoda, and K. Yoshida, Uniformly Porous MgTi2O5 with Narrow Pore-Size Distribution: XAFS Study, Improved In-Situ Synthesis, and New In-Situ Surface Coating, Adv. Eng. Mater, 14 [12] 1134-1138 (2012). |
||||||||||||||||
| 2012.9.24-25 Far East STEMinars for High school students from U.S. DoDEA and Meikei
High school Our labporatory offered a course of porous materials processing for USDoDEA High Schools and Meikei high school. Students experienced ceramics processing, analysis, and scientific presentation. |
||||||||||||||||
   
  |
||||||||||||||||
| http://www.dodea.edu/Pacific/Activities/STEMinars.cfm | ||||||||||||||||
| 科学研究費補助金 若手研究(A)プロジェクト (平成19~22年度) 「三次元ネットワーク型多孔質複合セラミックスのディーゼル粒子除去フィルターへの応用」 |
||||||||||||||||
| 本研究課題は、これまで私が行ってきた「三次元ネットワーク型多孔体に関する研究開発」をディーゼル粒子除去フィルター(DPF)に展開したものです。研究ターゲットは下記のとおりです。表は提案時点のものですので、実際のプロジェクトでは多少の変更が生じます**が、出来る限り「使える材料を作る」というコンセプトのもとで進めて行きたいと考えています。 | ||||||||||||||||
(詳細:PDF: 99 kB) |
||||||||||||||||
表1.三次元ネットワーク型多孔質複合材料(UPC-3D)をDPFに適用するための課題と研究内容
|
||||||||||||||||
| **初年度購入の高温X線回折装置に、予算の都合上、ICDD-JCPDFのデータベースを残念ながらつけることができませんでした。これがあると研究が大きくはかどりますので、財団助成などなどで何とかしたいと考えています。皆さん、是非応援してください! | ||||||||||||||||
| Y. Suzuki, and M. Morimoto, "Porous
MgTi2O5/MgTiO3 Composites with Narrow Pore-Size Distribution: In Situ
Processing and Pore Structure Analysis,"J. Ceram. Soc. Jpn., 118 [9] 819-822 (2010). Y. Suzuki and M. Morimoto, "Uniformly Porous MgTi2O5 with Narrow Pore-Size Distribution: In Situ Processing, Microstructure and Thermal Expansion Behavior," J. Ceram. Soc. Jpn., 118 [12] 1212-1216 (2010). Y. Suzuki and Y. Shinoda, "Magnesium Dititanate (MgTi2O5) with Pseudobrookite Structure: A Review," Sci. Tech. Adv. Mater., 12 [3] no.034301 (2011). |
||||||||||||||||
|
|
||||||||||||||||
| 科学研究費補助金 若手研究(A)プロジェクト (平成16~17年度) 「三次元ネットワーク型多孔質複合セラミックス膜の創製と新規複合塩多孔質前駆体の合成」 |
||||||||||||||||
本研究課題は、平成11年4月~平成15年4月にかけて、提案者らにより行われた、「三次元ネットワーク型多孔質セラミックス複合材料の創製」の研究成果を発展させたものです。 これまでバルク体で得られていた三次元ネットワーク多孔体のプロセッシング及び構造デザインを、精密ろ過フィルターや種々の電子デバイスに応用が可能な膜形態に発展させることを狙いとしています。さらに、所望の組成の三次元ネットワーク多孔体膜を合成するために必要な、気孔形成成分含有の複合塩前駆体合成を併せて行っていくことを予定しています。以下では、まず、Al2O3/REPO4系にUPC-3Dを拡張した例を紹介します。 また、薄膜化については、1D-Nano PJのページでも「色素増感太陽電池(DSC)応用」として一部紹介していますです。 |
||||||||||||||||
 |
Porous Al2O3/20 vol% LaPO4 and Al2O3/20 vol% CePO4 composites with very narrow pore-size distribution at around 200 nm have been successfully synthesized by reactive sintering at 1100oC for 2 h from RE2(CO3)3-xH2O (RE = La or Ce), Al(H2PO4)3 and Al2O3 with LiF additive. Similarly to the previously reported UPC-3Ds (uniformly porous composites with three-dimensional network structure, e.g. CaZrO3/MgO system), decomposed gases in the starting materials formed a homogeneous open porous structure with porosity of ~ 40 %. X-ray diffraction (XRD), 31P magic-angle spinning nuclear magnetic resonance (31P-MAS-NMR), scanning electron microscopy (SEM), and mercury porosimetry revealed the structure of the porous composites. | |||||||||||||||
 |
Porous ceramics are widely used as filters, lightweight structural components,
electrodes, catalysts, catalyst supports, bioreactors, and so on. The Al2O3/monazite
system is expected to be a good porous material due to its favorable properties
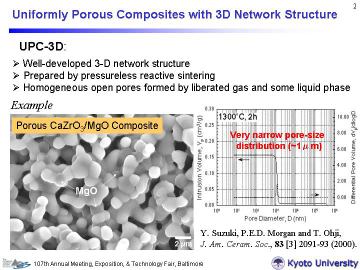
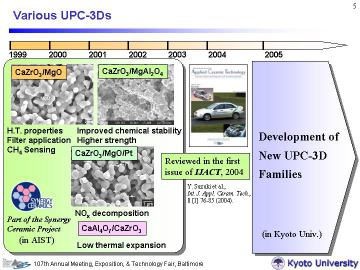
as described above. The present authors have recently developed uniformly porous CaZrO3/MgO and related composites with 3-D network structure (UPC-3D) via a pyrolytic reactive sintering process. The pore-size distribution was very narrow (with pore size of ~ 1 micrometer), and the porosity was controllable (typically ~30-50%) by changing the sintering temperature. |
|||||||||||||||
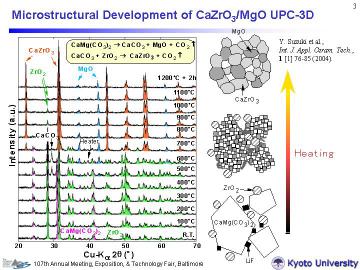 |
This process takes advantage of the evolved gas from a decomposing starting material and does not require any additional pore forming agent nor long-time burning-out process. Through liquid formation via mineralizer doping, strong necks are formed between constituent particles before completion of the pyrolysis, resulting in the formation of a strong 3-D network structure. |
|||||||||||||||
 |
||||||||||||||||
 |
||||||||||||||||
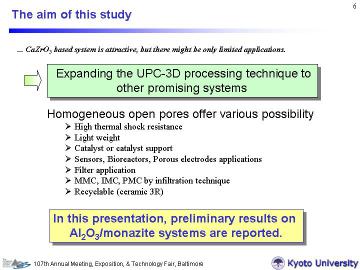 |
In this study, we have attempted to synthesize new porous Al2O3/20 vol% REPO4 (RE = La and Ce) in situ composites with narrow pore size distribution by the reactive pressureless sintering process. | |||||||||||||||
 |
||||||||||||||||
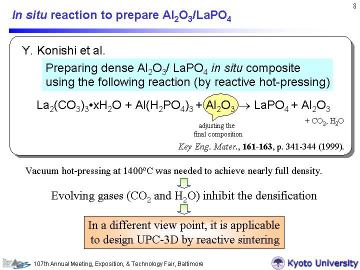 |
Konishi et al. have prepared dense Al2O3/LaPO4 by reactive hot-pressing
in vacuum, using the following in situ reaction: La2(CO3)3oxH2O + Al(H2PO4)3 + Al2O3 -> LaPO4 + Al2O3 (+CO2 +H2O) In this reactive sintering system, alpha-Al2O3 was added to adjust the final composition. In their study, vacuum hot-pressing at 1400oC was required to remove evolved gases and to obtain high density. From a different viewpoint, however, this reaction can be applicable to design open-porous composites by using the evolved gases as a sort of pore forming agents. |
|||||||||||||||
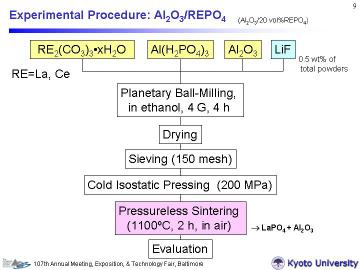 |
The RE2(CO3)3oxH2O, Al(H2PO4)3 and Al2O3 powders with LiF (0.5 mass% for total starting powders) were wet-ball milled in ethanol for 4 h in a planetary ball-mill (acceleration: 4 G). The mixed slurry was dried and then sieved through a 150-mesh screen. To obtain bulk porous composites, the mixed powders were cold isostatically pressed at 200 MPa after mold-pressing. The green compacts with no binder, 15 mm in diameter and ~ 3 mm in thickness (cylinder), were sintered in air at 1100oC for 2 h to obtain the porous composites. |
|||||||||||||||
 |
To determine the H2O content in commercial hydrated RE carbonate powders,
TG-DTA experiments were conducted. Left figure shows TG-DTA curves for
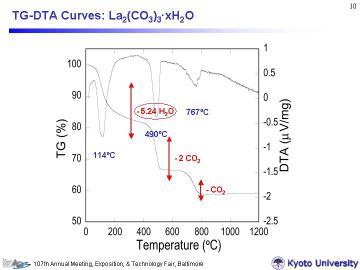
(a) La2(CO3)3-xH2O and (b) Ce2(CO3)3-xH2O powders, respectively. For the La-system, adsorbed and hydrated H2O (equivalent to 5.2 molecules) evaporated at less than ~200oC, then two molecules of CO2 was emitted with a peak at 489.2oC to form La2O2CO3 intermediate, and finally one molecule of CO2 was released with a peak at 766.8oC to form La2O3. Thermal decomposition finished at ~800oC. |
|||||||||||||||
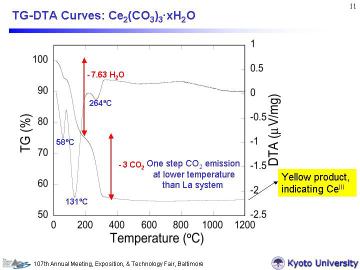 |
For the Ce-system, adsorbed and hydrated H2O (~8 molecules) evaporated
less than ~200oC, then three molecules of CO2 was emitted with a peak at 264.9oC (apparently in one-step) to form cerium oxides. Thermal decomposition finished at ~300oC. CeIII in cerium oxides is unstable in an oxidative atmosphere and tends to be oxidezed into CeIV. (However, once CePO4 is formed under reactive sintering, Ce ions become trivalent). For the Ce-system, precise determination of the H2O amount by TG-DTA analysis is a littre more difficult than for the La-system. The obtained compositions, i.e., La2(CO3)3-5.2H2O and Ce2(CO3)3-8H2O, were used for the powder mixing process. |
|||||||||||||||
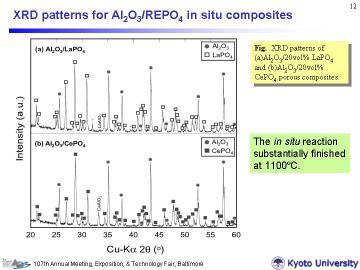 |
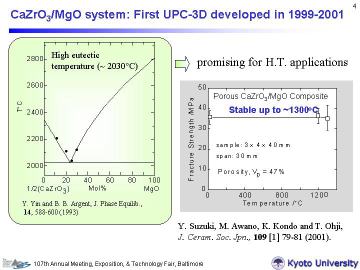
Sintered samples are crack free and almost the same size as green bodies, which implies the applicability of near net shaping. Left figure shows XRD patterns of the bulk porous composites. For both systems, main components were alpha-Al2O3 and monazite phases, as expected. Some LaAlO3 or CeAlO3 formation indicates the existence of excess La or Ce in the composites. This may be attributed to some existence of H2O in the Al(H2PO4)3 powder, in other words, the actual content of PO43- was slightly less than the ideal stoichiometry. Considering high-temperature applications, however, some excess of rare earth elements is favored to avoid the formation of AlPO4. |
|||||||||||||||
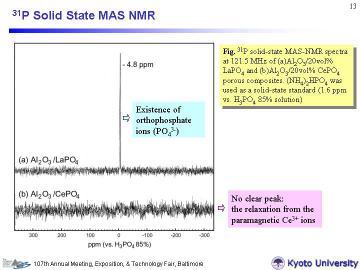 |
Left figure represents 31P MAS-NMR spectra for (a)Al2O3/20vol% LaPO4 and (b)Al2O3/20vol% CePO4 porous
composites. For the Al2O3/LaPO4 system, only a sharp peak at -4.8 ppm was observed. This result means the P atoms in the composite exist as orthophosphate ions (PO43-), and the ppm value is close to reported one for monoclinic LaPO4 (-4.5 ppm).18 On the other hand, for the Al2O3/CePO4 system, no clear peak was observed. This phenomenon can be well-explained by the relaxation from the paramagnetic Ce3+ ions, as reported by Xu et al. Hence, this NMR spectrum is an indirect evidence for the CeIII orthophosphate. |
|||||||||||||||
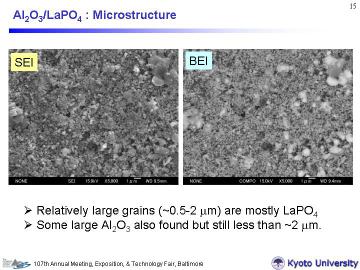 |
Left figures demonstrates typical microstructure of Al2O3/20vol% LaPO4
(SEI and BEI images). The composites consisted of about 100-200 nm of Al2O3 grains and about 1-2 micrometer of REPO4 grains (confirmed by EDS and backscattered electron image). The porous composites had uniform porous structure with open-pore network, similarly to the reported UPC-3Ds. The open pore-size were estimated around 200 nm from these figures. |
|||||||||||||||
 |
||||||||||||||||
 |
||||||||||||||||
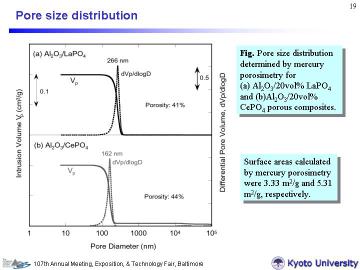 |
To quantitatively evaluate the pore structure, mercury porosimetry measurements were conducted. Left figure shows the total pore volume (intrusion volume) and the pore-size distribution of the two composites. Although the Ce-system contained somewhat smaller pores than the La-system, the profiles were very similar to each other. The composites (porosity: ~ 40%) had very narrow pore-size distribution with a peak size at 266 nm for La-system and 162 nm for Ce-system, in good agreement with the SEM observation. Formation of smaller pores in the Ce-system can be explained by the earlier termination of the pyrolysis of starting carbonate; for the Ce-system, evolved CO2 from carbonate (acting as pore forming agent) probably escaped at the earlier stage of reactive sintering. In addition, Al2O3 grains in the Ce-system were slightly smaller than those in the La-system. | |||||||||||||||
 |
Porous Al2O3/LaPO4 and Al2O3/CePO4 composites were synthesized by the reactive sintering at 1100oC for 2 h from RE2(CO3)3oxH2O (RE = La or Ce), Al(H2PO4)3 and Al2O3 with LiF additive. SEM observation and mercury porosimetry showed that the porous Al2O3/LaPO4 and Al2O3/CePO4 composites had very narrow pore-size distribution at about 200 nm. |
|||||||||||||||
|
|
||||||||||||||||
| 新規セラミックス多孔体の開発 (シナジーセラミックスプロジェクト) | ||||||||||||||||
|
1999年4月から2002年3月までの3年間、シナジーセラミックスプロジェクトの一環として行った仕事です。私の工業技術院名古屋工業技術研究所(その後、産業技術総合研究所)でのメインテーマのひとつです。これが現在のUPC-3Dプロジェクトにつながっています。高温特性や流体透過性に優れた新規のセラミックス多孔体を開発を目指したもので、非常に均質な3次元ネットワーク構造をもつ多孔体を開発することができました。環境や人体に対する影響も小さく、低コストで安全な材料です。バルク状の多孔体中には約1ミクロンの非常に均一な開気孔が分散しています。以下では当時の新聞記事を紹介します。 2001(平成13年)/3/6 日経産業新聞(10面) 2001(平成13年)/2/25 化学工業時報(1-2面) 2000(平成12年)/4/12 化学工業日報(11面) 2000(平成12年)/3/29 日本工業新聞(31面) 2000(平成12年)/3/29 日経産業新聞(5面) |
||||||||||||||||
|
|
||||||||||||||||
| Copyright (c) 2011-2012 Yoshikazu Suzuki Laboratory, University of Tsukuba |
||||||||||||||||