Welcome to Tsujimura laboratory
All bio-energy to sustain life on the earth is converted from
the energy of redox reactions, which involve transfer of electrons and ions.
“Bioelectrochemistry” is a new interdisciplinary field to study chemistry and
mechanism of electrons and ions transfer at the level of molecule.
Our group, launched on August 2011, focuses on research at the frontiers of knowledge of enzymes,
mediators, surfaces and immobilization strategies aimed at improving enzymatic electron
transfer reactions for application towards biofuel cells, that generates electricity
from carbohydrates (sugar) utilizing enzymes as its electrocatalysts, and integrated
biosensing systems for environment, diagnosis and healthcare.
Keywords
bioelectrochemistry, electrochemistry, redox enzyme, biofuel cell, biosensor, mediator, direct electron transfer, mediator, mediated electron transfer, oxidase, dehydrogenase, porous carbon, carbon gel, carbon cryogel, bioelectrocatalysis, electrocatalyst
Leaflet
Contact information
You may contact the laboratory at the following postal or email addresses.
Postal address
Dr Seiya Tsujimura
Division of Materials Science, Faculty of Pure and Applied Sciences, University of Tsukuba
1-1-1 Tennodai, Tsukuba, Ibaraki, 305-8573, Japan
Email address
seiya (at) ims.tsukuba.ac.jp
Seiya Tsujimura, PhD
Associate Professor

Dr Seiya Tsujimura obtained his PhD on the topic of energy conversion systems based on bioelectrocatalytic reactions under the supervision of Professor Kenji Kano in March 2007 from Kyoto University. Dr Tsujimura received research fellowships from Japan Society for the Promotion of Science for Young Scientist during his doctoral course (from April 2002 to June 2003). He has been employed at Kyoto University as an assistant professor (permanent) from July 2003 to August 2011. He spend one year as a visiting researcher in CNRS Paul Pascal laboratory in Bordeaux (France) to develop novel enzymatic biofuel cells with Dr. Nicolas Mano (from February 2010 to December 2010). He took up a position as associate professor (permanent) at University of Tsukuba (August 2011) and heads the bioelectrochemistry laboratory. Prof. Tokuji Ikeda and Prof. Kenji Kano, who are the supervisors and also his mentor, are a worldwide authority in the field of bioelectrochemistry.
His research fields lie in the area of bioelectrochemistry, at the interface between electrochemistry and biological redox reactions. More specifically, his group employs engineered biocatalysts and nano-structured electrode materials to design new biosensing system with higher accuracy and biofuel cells with higher power denisty. His interest also focused on the fundamentals of redox enzymes for the better understanding of the redox flows in the microbe and for the development of enzyme electrode. The research of his group also focuses in the redox controlling of the microbe with the self-extracted redox molecules.
Prizes
Best faculty member, Univ Tsukuba (2023).
Shikata Medal, the polarographic society of Japan (2022).
JSBBA Award for Young Scientists (2018)
Best Poster Presentation Award at 62th International Society of Electrochemistry (2011).
Encouraging Prize at Enzyme Application Symposium (2008).
Best Poster Presentation Award at 56th International Society of Electrochemistry (2005).
Best Paper Award in The Electrochemical Society of Japan (2003).
Education/teaching
-Advanced Materials and Molecular Engineering
-Instrumental Spectroscopy
-Chemistry
-Life Science
-Introduction to Molecular Engineering
-Material Chemistry
Reserch Interests
Redox biological reactions, electrode reactions, and bioelectrocatalytic reactions, for the development of bioelectric devices, such as biofuel cells and biosensors, in cooperation with bioelectrochemistry, enzyme science, applied microbiology, surface chemistry, carbon science, organic chemistry, inorganic chemistry.
Evaluation of Chemical properties and catalytic function oxidoreductase, and studies on the enzyme electrode reaction
We study the relationship between the redox behavior (redox potential etc.) and catalytic activity of redox enzyme.
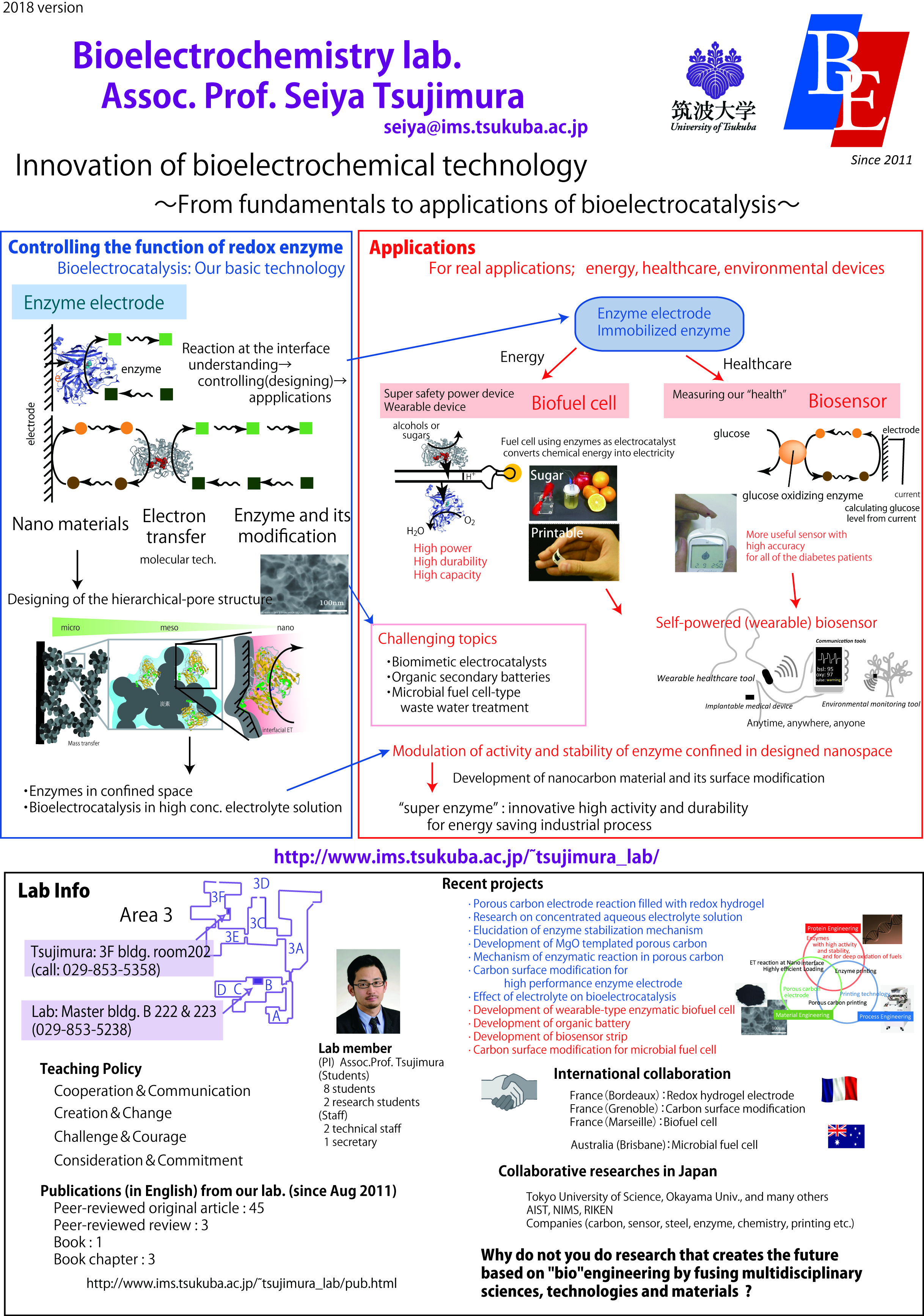
We are going to develop an enzyme electrode with high performance. Redox enzyme electrodes enable electrochemical oxidation of sugar and alcohol and reduction of oxygen under very mild conditions. We study the basics of bioelectrochemistry of redox enzyme and apply them to bioelectrochemical applications.
Application of enzyme electrode to biofuel cells and biosensors
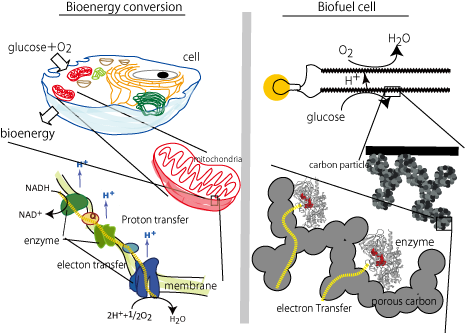
Biofuel cells (BFC) are devices for converting biochemical energy into electrical energy using enzyme as an electrocatalyst and have received considerable attentions as ubiquitous devices for energy demand because of their efficient and eco-friendly properties. They can utilize sugars and alcohols as fuel at ambient temperature and atmospheric pressure. In addition, the cell unit is composed of fuel oxidizing enzyme-modified anode and O2 reducing enzyme modified cathode. The simple structure would enable the BFC to be miniaturized. The enzyme-catalyzed electrode reaction is called bioelectrocatalysis, which is classified into two types: one is direct electron transfer type, in which the electron transfer occurs directly between enzymes and electrodes, and the other is mediated electron transfer type, in which electron transfer mediators transfer the electron between enzymes and electrodes. Dr. Tsujimura has developed suitable enzymes for bioelectrocatalytic reactions and fabricated enzyme electrode using a nano-structured carbon electrode for respective enzymes to develop a high-power biofuel cells. We are going to develop more powerful and tough biofuel cell.
Nano-structured materials and electro-enzymatic reaction in nano-porous electrode
We study the electron transfer reactions of redox enzymes adsorbed on the electrode; the effect of the chemical and structural interactions between the enzyme and the electrode. In addition, in order to improve the output power of biofuel cell, we are developing the porous carbon electrode with a high electrical conductivity and a contolled pore structure in the nanometer scale. To improve the durability and electron transfer rate of the enzymes, the mechanism of interfacial electron transfer reactions of the enzyme in the nano porous electrode are studied.
Electrochemical control of microbial metabolism and microbial fuel cells
Dr Tsujimura has studied to control the redox state of the microbial cells and to promote microbial growth (inhibition). He reported the electrochemical applications using microorganisms, such as microbial fuel cells, biosensors, and bioreactor. He focused on the electron transfer mechanism between the electrode and the intracellular microbial metabolic reactions. Although an artificial electron mediator is required to control the intracellular redox state, it is a major barrier for the practical usage. It was found that molecule redox excreted by microbes themselves can work as electron transfer mediator. Based on this finding, we will make the sustainable and environment-friendly microbial electrochemical devices.
Selected Papers (2001- 2010)
- Electrochemical reaction of fructose dehydrogenase on carbon cryogel electrodes with controlled pore sizes, Tsujimura S., Nishina A., Hamano Y., Kano K., and Shiraishi S., Electrochem. Commun., 12 (3), 446-449 (2010).
- Coulometric D-fructose biosensor based on direct electron transfer using D-fructose dehydrogenase, Tsujimura, S., Nishina, A., Kamitaka, Y., Kano, K. , Anal. Chem., 81, 9383-9387 (2009).
- Air Diffusion Biocathode with CueO as Electrocatalyst Adsorbed on Carbon Particle Modified Electrodes, Kontani. R., Tsujimura, S., Kano, K. , Bioelectrochemistry, 76 (1/2), 10-13 (2009).
- A High-power Glucose/oxygen Biofuel Cell Operating under Quiescent Conditions, Sakai, H., Nakagawa, T., Sato, A., Tomita, T., Tokita, Y., Hatazawa, T., Ikeda, T., Tsujimura, S., Kano, K. , Energy Environ. Sci., 2 (1), 133-138 (2009).
- Direct Electrochemistry of CueO and Its Mutants at Residues to and near Type I Cu for Oxygen-Reducing Biocathode, Miura, Y., Tsujimura, S., Kurose, K., Kamitaka, Y., Kataoka, K., Sakurai, T., and Kano, K. , Fuel Cells, 9(1), 70-78 (2009).
- CueO-immobilized Porous Carbon Electrode Exhibiting Improved Performance of Electrochemical Reduction of Dioxygen to Water, Tsujimura, S., Miura, Y., Kano, K. , Electrochim. Acta, 53 (18), 5716-5720 (2008).
- Diffusion-controlled Oxygen Reduction on Multi-copper Oxidase-adsorbed Carbon Aerogel Electrodes without Mediator, Tsujimura, S., Kamitaka, Y., and Kano, K., Fuel cells, 7 (6), 463-469 (2007).
- Fructose/dioxygen biofuel cell based on direct electron transfer-type bioelectrocatalysis, Kamitaka, Y., Tsujimura, S., Setoyama, N., Kajino, T., and Kano, K. , Phys. Chem. Chem. Phys., 9 (15), 1793-1801 (2007).
- Potential-step coulometry of D-glucose using novel FAD-dependent glucose dehydrogenase, Tsujimura, S., Kojima, S., Ikeda, T., Kano, K., Anal. Bioanal. Chem., 386 (3), 645-651(2006).
- Novel FAD-dependent glucose dehydrogenase to construct dioxygen-insensitive glucose biosensor, Tsujimura, S., Kojima, S., Kano, K., Ikeda, T., Sato,M., Sanada, H., and Omura, H. , Biosci., Biotech., Biochem., 70 (3), 654-659 (2006).
- Mediated Spectroelectrochemical Titration of Proteins for Redox Potential Measurements by a Separator-less One-compartment Bulk Electrolysis Method, Tsujimura, S., Kuriyama, A., Fujieda, N., Kano, K., and Ikeda, T. , Anal. Biochem., 337 (2), 325-331 (2005).
- Bilirubin Oxidase in Multiple Layer Catalyzes Four-electron Reduction of Dioxygen to Water Without Redox Mediators, Tsujimura, S., Kano, K., and Ikeda, T. , J. Electroanal. Chem., 576 (1), 113-120 (2005).
- Kinetic Study of Direct Bioelectrocatalysis of Dioxygen Reduction with Bilirubin Oxidase at Carbon Electrodes, Tsujimura, S., Nakagawa, T., Kano, K., and Ikeda, T., Electrochemistry, 72 (6) 437-439 (2004).
- Mediated Bioelectrocatalytic O2 Reduction to Water at Highly Positive Electrode Potentials near Neutral pH, Tsujimura, S., Kawaharada, M., Nakagawa, T., Kano, K., and Ikeda. T., Electrochem. Commun., 5 (2), 138-141 (2003).
- Bioelectrocatalysis-based Dihydrogen/Dioxygen Fuel Cell Operating at Physiological pH, Tsujimura, S., Fujita, M., Tatsumi, H., Kano, K., and Ikeda, T., Phys. Chem. Chem. Phys., 3(3), 1331-1335 (2001).
- Bioelectrocatalytic Reduction of Dioxygen to Water at Neutral pH Using Bilirubin Oxidase as an Enzyme and 2,2'-Azinobis (3-Ethylbenzothiazolin-6-Sulfonate) as an Electron Transfer Mediator, Tsujimura, S., Tatsumi, H.,Ogawa, J., Shimizu, S., Kano, K., and Ikeda, T., J. Electroanal. Chem., 496 (1/2), 69-75 (2001).
- Click for more information (Japanese page)