Air-gap micro ammonia electrode and integrated sensing system for creatinine and urea
We developed a fast-responding Severinghaus-type ammonia gas-sensing electrode by using an air gap formed in a flow channel (Fig.1). The air gap was maintained with a hydrophobic layer of silicone rubber intercalated between two glass substrates. An internal filling solution of the ammonia gas-sensing electrode was separated from a sample solution by an air gap. When a sample solution is introduced into the flow channel, ammonia dissipates through the air gap into the internal filling solution of the electrode. The accompanying pH change was measured with a thin-film pH-indicator electrode.
Because of the air gap, the response was significantly faster than the electrodes of the conventional structure. The 90% response time was less than 1 min for an ammonia concentration greater than 1 mM. A linear relationship was observed in the calibration curve, and the lower detection limit was 20 μM.
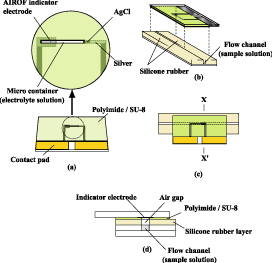
Fig. 1 Construction of the ammonia gas-sensing electrode and the creatinine-sensing system. (a): plan view of the substrate with the detecting electrodes for the ammonia gas-sensing electrode. (b): plan view of the substrate with the flow channel. (c): cross-section of the completed structure of the ammonia gas-sensing electrode constructed from (a) and (b) viewed from the glass side. (d): magnified cross-section of (c) along the line X-X’.
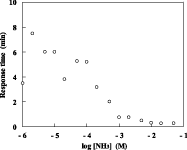
Fig. 2 Dependence of the 90% response time on the concentration of ammonia at 37 degree.
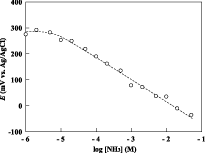
Fig.3 Calibration curve for the ammonia gas-sensing electrode at 37 degree.